Boron Silicon Germanium Arsenic Antimony Tellurium. 12 protons and 14 neutrons.
Periodic Table Of The Elements Metalloids
When boron reacts with sodium it acts as a non-metal whereas in case of reaction with fluorine boron exhibits metallic properties.

. A Si b Sc c V d Sn 3What is the most abundant element in the Earths crust. The elements on the periodic table can be grouped as metals nonmetals or metalloids. All of the metalloids have some properties of both metals and nonmetals and are located directly in between.
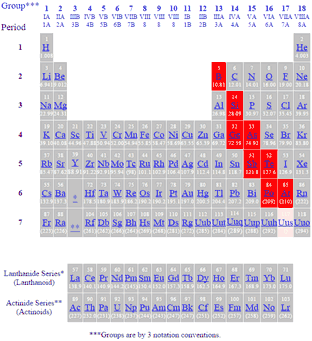
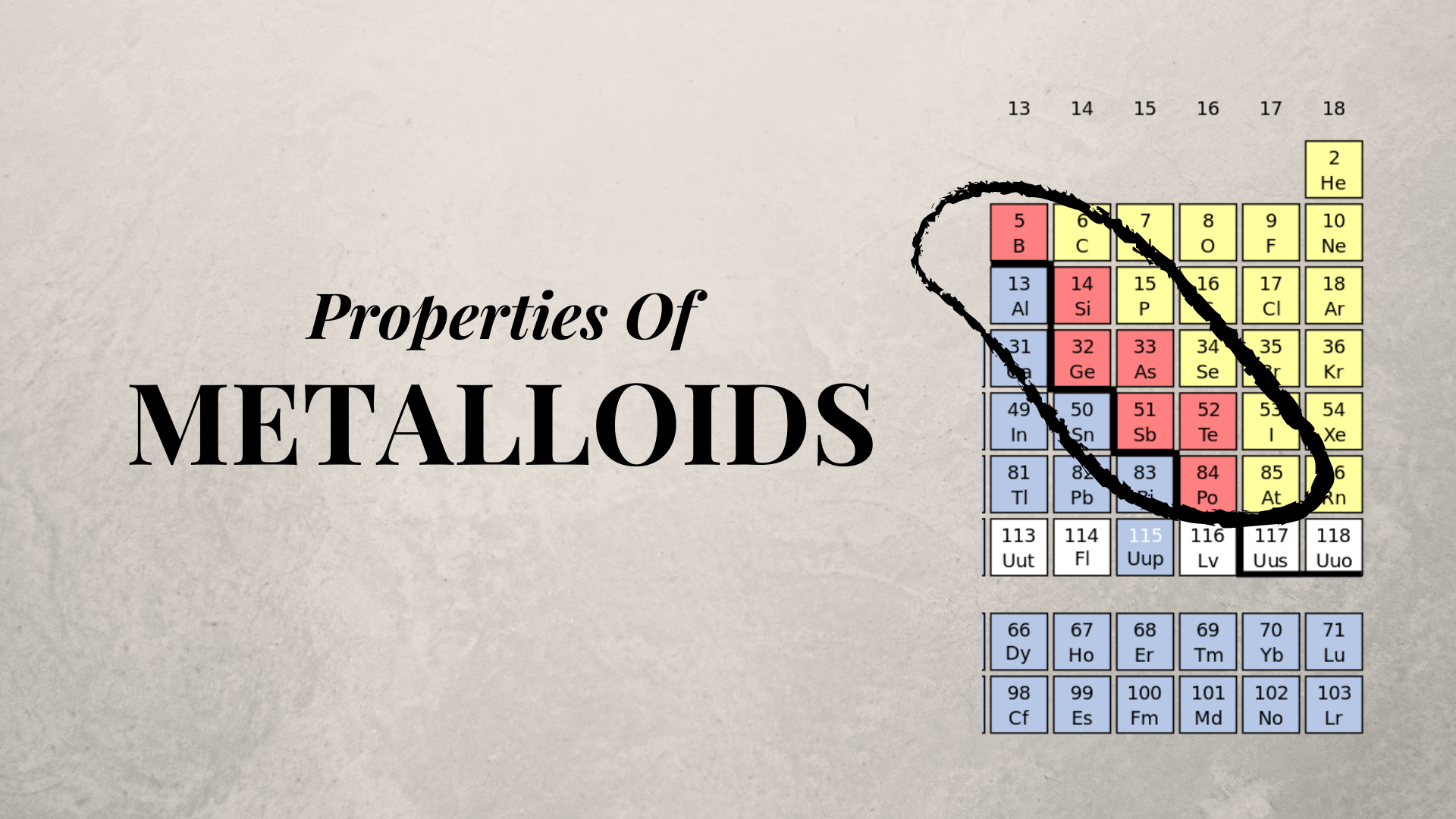
Metalloids can also be called semimetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Astatine polonium tellurium antimony arsenic germanium silicon boron.
Arsenic As is the metalloid andall the remaining are non-metals. AskedApr 25in Chemistryby chetnaMohanty438kpoints ntse. 1 K Mn As Ar 2 Li Mg Ca Kr 3 Ba Ag Sn Xe 4 Fr.
Boron B Silicon Si Germanium Ge Arsenic As Antimony Sb Tellurium Te Polonium Po. However the following elements are generally considered to be metalloids or semimetals. It was supposed to classify Astatine as either a non-metal or metalloid.
An atom that has an atomic number of 12 and a mass number of 26 is an isotope of an atom that has. An atom that has an atomic number of 12 and a mass number of 26 is an isotope of an atom that has. A Fe b C c O dN 4What is the name given to elements that exist in different forms such as graphite diamond and.
Compare the first ionization energy of sodium to that of potassium. Metalloids include Boron Silicon Germanium Arsenic Antimony Tellurium and Polonium. The element is named after the Lawrence Livermore National Laboratory in the United States which collaborated with the Joint Institute for Nuclear.
It is an extremely radioactive element that has only been created in the laboratory and has not been observed in nature. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Name two elements that have similar properties to those of the element potassium.
The arrangement or exact location of electron in an atom. A unique platform where students can interact with teachersexpertsstudents to get solutions to their queries. A unique platform where students can interact with teachersexpertsstudents to get solutions to their queries.
The most commonly recognised metalloids are boron silicon germanium arsenic antimony and tellurium. Welcome to Sarthaks eConnect. What are the 9 metalloids.
Semimetals or metalloids are chemical elements that have properties of both metals and nonmetals. The element carbon is a non-metal but graphite displays limited conductivity which is the characteristic of a metalloid. These elements are germanium and arsenic.
Silicon and germanium exhibit properties of a semiconductor. An atomic number of 12 and a mass number of 24. 1 Boron 2 Sodium 3 Chlorine 4 Aluminium.
Metalloids are important semiconductors often used in computers and other electronic devices. These elements are germanium and arsenic. A Ge bS c Be dAi What is the symbol for the element in the third period and the fourth group.
Which set of elements contains a metalloid. This slice of the periodic table highlight the elements classified as metalloids. Livermorium is a synthetic chemical element with the symbol Lv and has an atomic number of 116.
1 Boron 2 Sodium 3 Chlorine 4 Aluminium. Any group 1A element The energy of sodium is greater than potassium. AskedApr 25in Chemistryby chetnaMohanty438kpoints ntse.
Choose the metalloid from the following elements. Notice that aluminum borders the line but it is considered to be a. Choose the metalloid from the following elements.
Find step-by-step Chemistry solutions and your answer to the following textbook question. The six commonly recognised metalloids are boron silicon germanium arsenic antimony and. Despite the lack of specificity the term remains in use in the literature of chemistry.
On the periodic table the elements colored yellow which generally border the stair-step line are considered to be metalloids. The six elements that are unanimously considered to be metalloids are the following. 12 neutrons and 14 protons.
Boron B Silicon Si Germanium Ge Arsenic As Antimony Sb Tellurium Te Polonium Po These seven elements were classified as metalloids in the periodic table from the 13th to the 16th group. There are two metalloids in period 4 in the Periodic Table of elements. 201 rows The elements commonly classified as metalloids are boron silicon germanium.
Following are the elements that are considered to be metalloids. Welcome to Sarthaks eConnect. Which of the following elements is a metalloid.

Metalloids Chemistry For Non Majors
4 Properties Of Metalloids Science Trends


0 Comments